Consider the Resonance Structures of Formate.
Each oxygen atom has a double bond 50 of the time. Each oxygen atom has a double bond 50 of the time.
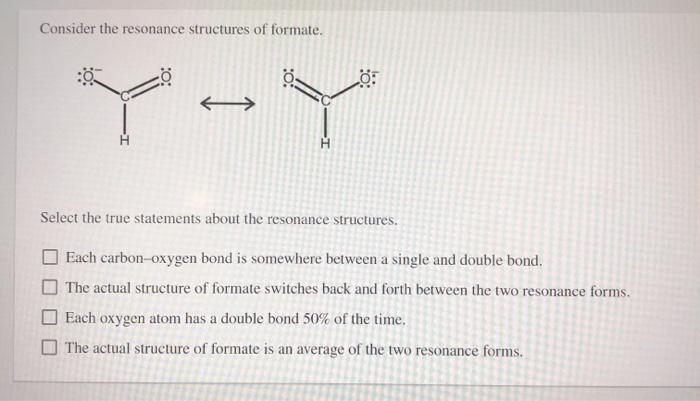
Solved Consider The Resonance Structures Of Formate O اس Chegg Com
The actual structure of formate is an average of the two resonance forms.

. The actual structure of formate is an average of the two resonance forms. The first lewis structure of formate has a central carbon atom. Science Chemistry QA Library Consider the resonance structures of formate.
Each carbon-oyxgen bond is somewhere between a single and double bond. The actual structure of formate is an average of the two resonance forms. The first Lewis structure of formate has a central carbon atom.
One of the bonds between carbon and oxygen is a single bond and the other bond is a double bond. Select the true statements about the resonance structures. Check all that apply.
Each carbon-oxygen bond is somewhere between a single and double bond. -Each oxygen atom has a double bond 50 of the time. Select the true statements about the resonance structures.
Each oxygen atom has a double bond 50 of the time. Consider the resonance structures of formate. A hydrogen atom an.
Consider the resonance structures of formate. -The actual structure of formate is an average of the two resonance forms. The actual structure of formate is an average of the two resonance forms.
Each carbon-oxygen bond is somewhere between a single and double bound. Each oxygen atom has a double bond 50 of the time. Each carbon-oxygen bond is somewhere between a single and double bond.
The single bonded oxygen has three lone pairs. In such cases resonance structures are used to describe chemical bonding. The actual structure of formate is an average of the two resonance forms.
Consider the resonance structures of formate. In many cases a single Lewis structure fails to explain the bonding in a moleculepolyatomic ion due to the presence of partial charges and fractional bonds in it. O The actual structure of formate is an average of the two resonance forms.
Select the true statements regarding these resonance structures of formate. D two osygen atoms are bonded to the carbon atom. Consider the resonance structures of formate.
Each carbon-oxygen bond is somewhere between a single and double bond. One of the bonds between carbon and oxygen is a single bond and the other bond is a double bond. The actual structure of formate switches back and forth between the two resonance fo.
The actual structure of formate switches back and forth between the two resonance forms. Select the true statements about the resonance structures. The actual structure of formate switches back and forth between the two resonance forms.
-Each carbon-oxygen bond is somewhere between a single and double bond. One of the bonds between carbon and oxygen is a single bond and the other bond is a double bond. A hydrogen atom and two osygen atoms are bonded to the carbon atom.
Onsider the resonance structures of formate. TY Select the true statements about the resonance structures. Each oxygen atom has a double bond 50 of the time.
Select the true statements about the resonance structures. The first Lewis structure of formate has a central carbon atom. The actual structure of formate is an average of the two resonance forms.
Each oxygen atom has a double bond 50 of the time. Each oxygen atom has a double bond 50 of the time. Each carbon oxygen bond is.
-The actual structure of formate switches back and forth between the two resonance forms. Every bond between carbon and oxygen within a resonance structure of formate is between a single and double bound while the actual structure of formate is the average of the two resonance forms and thus we note that the first and fourth statement are true. Consider the resonance structures of formate.
One of the bonds between carbon and oxygen is a single bond and the other bond is a double bond. The actual structure of formate is an average of the two resonance forms. The bond between carbon and hydrogen is a single bond.
The first lewis structure of formate has a central carbon atom. A hydrogen atom and two osygen atoms are bonded to the carbon atom. Each carbon-oxygen bond is somewhere between a single and double bond.
Select the true statements regarding the resonance structures of formate. Select the true statements about the resonance structures. The bond between carbon and hydrogen is a single bond.
2 See answers Advertisement Answer Expert Verified 50 5 3. A hydrogen atom and two osygen atoms are bonded to the carbon atom. The bond between carbon and hydrogen is a single bond.
Select the true statements about the resonance structures. Consider the resonance structures of formate. Each carbon-oyxgen bond is somewhere between a single and double bond.
The actual structure of formate switches back and forth between the two resonance forms. The actual structure of formate switches back and forth. Consider the resonance structures of formate.
Consider the resonance structures of formate. H Select the true statements about the resonance structures. Resonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule.
Each oxygen atom has a double bond 50 of the time. Each carbon-oxygen bond is somewhere between a single and double bond. The bond between carbon and hydrogen is a single bond.
The actual structure of formate switches back and forth between the two resonance forms. The single bonded oxygen has.
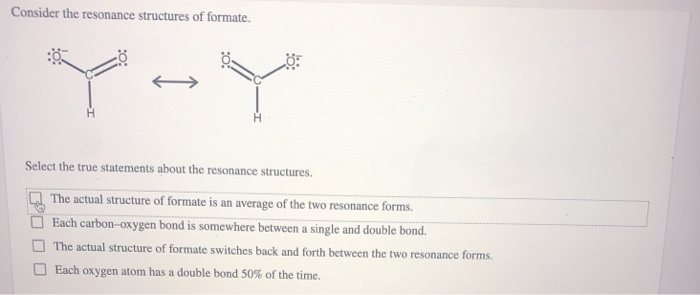
Solved Consider The Resonance Structures Of Formate Y Y Chegg Com
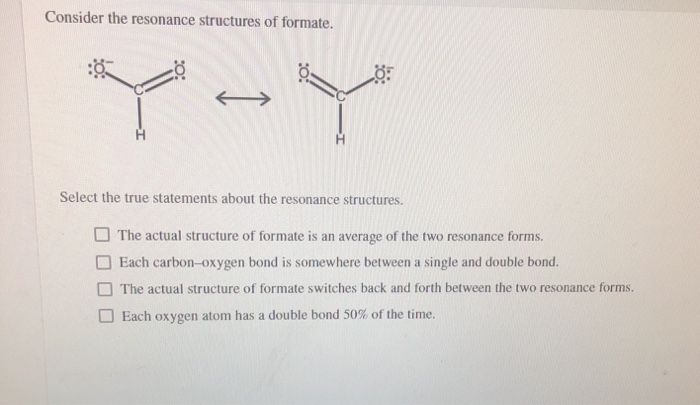
Solved Consider The Resonance Structures Of Formate Select Chegg Com
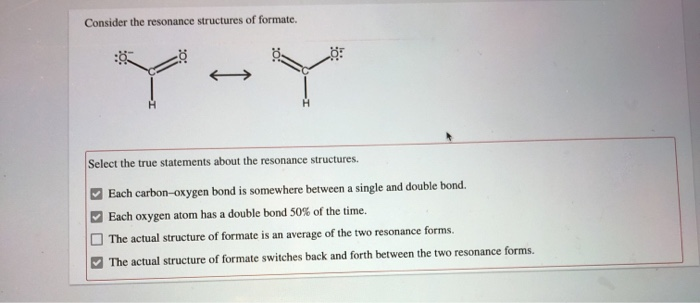
Solved Consider The Resonance Structures Of Formate Y Chegg Com
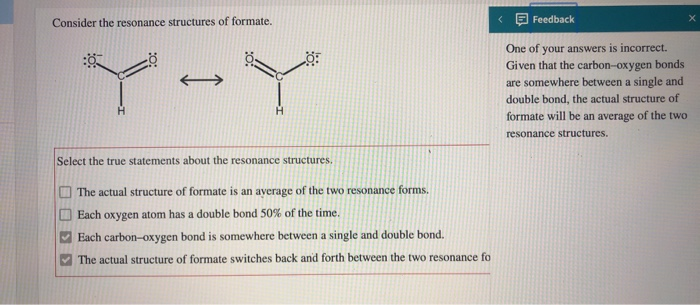
Solved Consider The Resonance Structures Of Formate Chegg Com
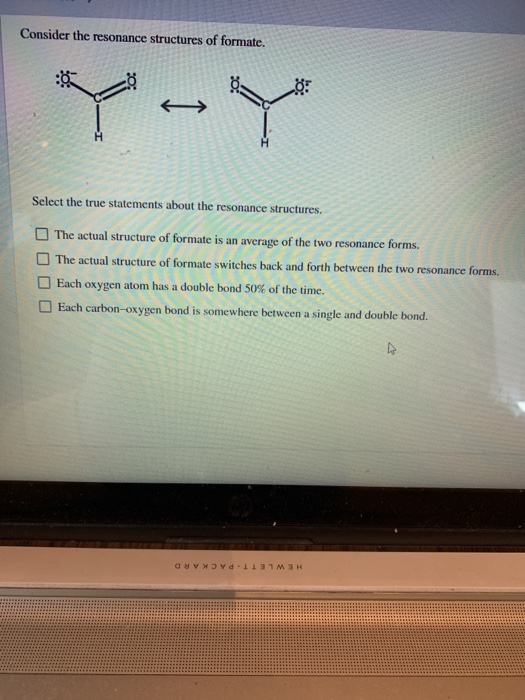
Solved Consider The Resonance Structures Of Formate Quy Y Chegg Com
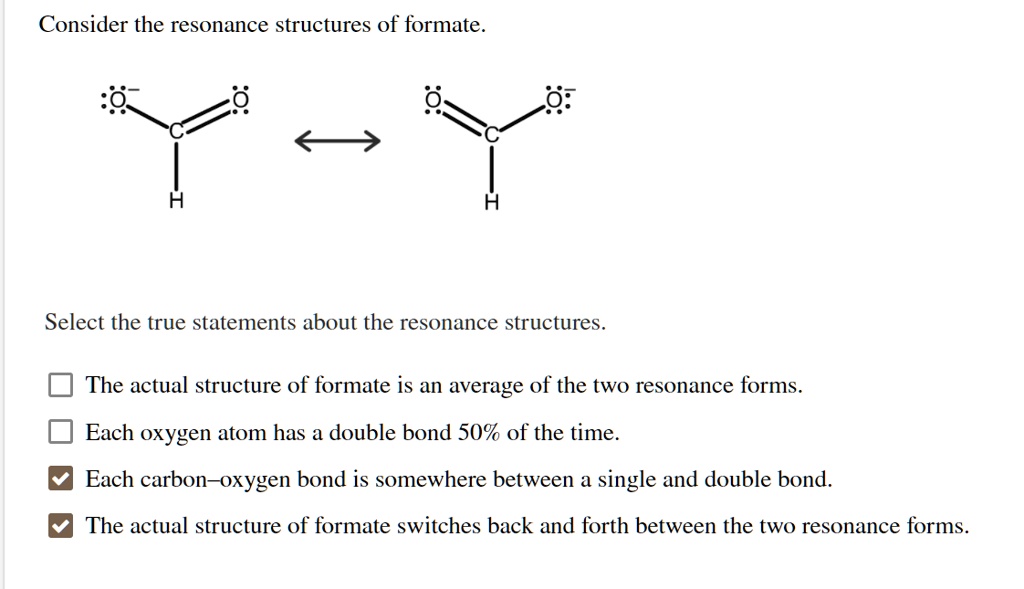
Solved Consider The Resonance Structures Of Formate Select The True Statements About The Resonance Structures The Actual Structure Of Formate Is An Average Of The Two Resonance Forms Each Oxygen Atom Has A
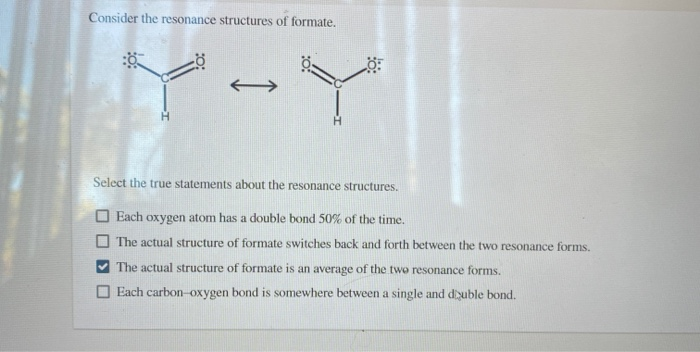
Solved Consider The Resonance Structures Of Formate H Chegg Com

Regiochemistry In E2 Reactions Of Cyclohexanes Organic Chemistry Organic Chemistry Study Chemistry Lecture
Comments
Post a Comment